Motor Oil Additives - Select Synthetics - AMSOIL Authorized Dealer
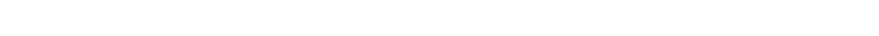


Main menu:
- Home Page
-
Products
-
AMSOIL Products
- Shop by Product
- Shop by Equipment
- Shop by Category
-
Product Lookup Guides
- Product Lookup Guides - Home
- Filter Lookup Guides
- Auto & Light Truck Lookup Guide
- Motorcycle & Dirt Bike Lookup Guide
- Harley-Davidson Products Guide
- All-Terrain Vehicle Lookup Guide
- Utility Terrain Vehicle Lookup Guide
- Snowmobile Lookup Guide
- Marine Outboard Lookup Guide
- Personal Watercraft Lookup Guide
- Small Engine Lookup Guide
- Free Product Catalog
- AMSOIL - The Blog
- AMSOIL YouTube Videos
- AMSOIL Performance Tests
- The AMSOIL Newsstand
- AMSOIL Testimonials
- AMSOIL Guarantee
- Safety Data Sheets
- Oil Analysis Services
- Shipping & Product Return
- Safe & Secure Shopping
-
AMSOIL Products
- About AMSOIL
-
Oil Basics
- What Is Motor Oil?
- What Is Oil Viscosity?
- What Does Motor Oil Do?
- Why Does Oil Need To Be Changed?
- How Often Should I Change My Oil?
- When Should I Do My 1st Oil Change?
- Can I Mix Different Oils?
- Lubrication Regimes
- Newtonian vs. Non-Newtonian
- Base Oil Groups
- Motor Oil Specifications
- Service Classifications & Grades
- Oil Evaluation Tests
- Oil Filtration
- What is Oil Analysis?
- Why Synthetics?
- Buy Wholesale
- FAQs
- Contact Us
Oil Basics > What Is Motor Oil?


What Is Motor Oil? – Part 3
Motor Oil Additives
When a Motor Oil is blended, additives/chemicals need to be added to the base oil (or oils) in order to make the finished product suitable for use in a modern engine.
Motor Oil Additives impart new characteristics to the base oil, or improve existing characteristics, enabling it to function in a desired manner when used to lubricate an engine. Base oils themselves perform most of the functions of lubricants, but they can only do part of the job. Additives are needed when a lubricant’s base oil doesn’t provide all the properties that a specific application requires.
Additives improve the overall performance of the engine oil. Their efectiveness depends both on the base oil(s) that they are added to and on how they interact with other chemicals/additives in the oil. An additive that works well in one oil might not in another. Therefore, it's important to understand how additives may interact with each other in synergistic or antagonistic ways when creating additive packages. Synergism gives the opportunity to use less overall additives. But antagonistic effects may prevent the use of certain types, and more may be needed to overcome the antagonistic effect.
Additives are used to enhance the beneficial properties of the base oil and minimize its detrimental properties. They help the base oil compensate for the deficiencies that are inherently present in it and help it stand up to extreme operating environments. Even the best base oil would not be able to protect as well against the effects of heat, shearing forces, chemical, fuel and water dilution, corrosion and wear particles. In short, additives make good base oils even better.
Additives are combined into well-balanced, optimized packages that allow the lubricant to meet a specified performance criteria in the finished fluid. One example of an additive package is the Dispersant Inhibitor (DI) package used for engine oils, containing primarily dispersants, detergents, oxidation inhibitors, antiwear agents and friction modifiers. Additives interact with each other and may have multi-functional properties. Each additive package is a unique blend whose combination produces a complex chemistry with positive and negative interactions.
Example of Dispersant Inhibitor (DI) additive package for motor oils (Figure courtesy of Vanderbilt Chemicals LLC.)
When using oil additives, more is not always better. As more additives are blended into the oil, sometimes there aren’t any additional benefits gained, and at times the performance actually deteriorates. In other cases, the performance of the additive doesn’t improve, but the duration of service (how long the oil can remain in service) does improve.
In addition, increasing the percentage of a certain additive may improve one property of an oil while at the same time degrade another. When the specified concentrations of additives become unbalanced, overall oil quality can also be affected. Some additives compete with each other for the same space on a metal surface. For example, if a high concentration of an anti-wear agent is added to the oil, the corrosion inhibitor may become less effective. The result may be an increase in corrosion-related problems. (See: “Can I Mix Different Oils?”)
It’s important to note that most additives are also sacrificial. Once they are gone, they’re gone. Having an oil analysis test done on the lubricant can determine the health/amount of the additives remaining in it.
Surface Active Additives
Surface active additives reduce friction and wear at the interface of rubbing surfaces.
Friction Modifier (FM), Anti-wear (AW) and Extreme-Pressure (EP) additives all form adsorbed layers of surface films that prevent surface asperity contact, reducing fatigue and corrosive wear each in different ways. Generally FM additives function by reversible or pseudo reversible adsorption processes. AW and EP additives chemically alter metal surfaces, forming strong, glass-like elastic materials that perform AW or EP functions.
Friction Modifiers
When two moving metal surfaces come in contact with each other, heat is generated by means of friction and pressure, inevitably leading to excessive wear, a decrease in fuel economy and can ultimately lead to premature engine failure.
Friction Modifiers alter the frictional properties of a lubricant and are used to give it more 'slippery' characteristics.
Properly selected friction modifiers can significantly reduce the coefficient of friction in boundary and mixed-film lubrication conditions (see Lubrication Regimes), preventing excessive wear and scoring, and improving fuel economy.
Friction Modifier Additives, and mild anti-wear agents, are polar molecules added to lubricants for the purpose of minimizing light surface contacts (sliding and rolling) that may occur in a given machine design and increase the oil’s lubricity. These are also called boundary lubrication additives.
These molecules have a polar end (head) and an oil-soluble end (tail). Once placed into service, the polar end of the molecule finds a metal surface and attaches itself. If you could see the orientation of the molecules on the surface, it would appear something like the fibers of a carpet, with each molecule stacked vertically beside the others.
As long as the frictional contact is light, these molecules provide a cushioning effect when one of the coated surfaces connects with another coated surface. If the contact is heavy, then the molecules are brushed off, eliminating any potential benefit of the additive.
Anti-Wear Agents
When the machine designer anticipates more than light surface contact (from shock loading, for instance), then he would select a stronger type of friction modifier characterized as an anti-wear agent.
Anti-wear agents chemically react with the metal surfaces during operation at elevated temperatures to form a film barrier between moving parts that helps prevent metal-to-metal contact.
AW additives work specifically to protect metal surfaces during boundary lubrication conditions. They form a ductile, ash-like sacrificial film at moderate to high contact temperatures reducing wear. Under boundary conditions, the AW film shears instead of the surface material.
In reacting with the metal surface, these additive types form new compounds such as iron chlorides, iron phosphides and iron sulfides (dependent upon which compound is used). The metal salts produce a chemical (soap-like) film that acts as a barrier to reduce friction, wear and metal scoring, and eliminate the possibility of ‘welding’.
Zinc dialkyldithiophosphate (ZDDP) is a common anti-wear agent. This type of additive literally reacts with the metal surface when the reaction energy (temperature) is high enough. The reaction layer provides sacrificial surface protection.
Note: Anti-wear agents perform in a similar manner to Extreme-Pressure agents, but tend to operate under lower loads, temperatures, and pressures.
Extreme-Pressure Agents
Extreme-Pressure agents coat metal surfaces to help prevent close-contact components from scoring and seizing (welding) under extreme heat and pressure. They are activated by high temperatures and high loads to react with the metal’s surface to form a sacrificial wear layer or film on components.
Extreme-Pressure agents are primarily used in the formulation of industrial gear lubricants. They are also used in load bearing greases, power-transmitting fluids and metalworking fluids.
They can be either sulfur based (DMTD derivatives, sulfurized olefins, sulfurized fats and oils and dithiocarbamates) or molybdenum based (dithiocarbamates, dithiophosphates). They are usually supplemented with anti-wear additives to make them effective across a wide range of conditions.
The rate of reaction of EP additives is greatest where the contact temperatures are highest; therefore, some difficulties are experienced in low-temperature applications when operating temperatures do not become high enough to fully activate the reactive EP agents. As the loading and metallic contact increase, the strength of the additive and reaction process increases.
The EP additives form organo-metallic salts on the loaded surfaces that serve as sacrificial films to protect against aggressive surface damage. One of the most popular types of EP chemicals used in the formulation of gear oils are sulfur-phosphorus based EP additives. However, sulfur‑phosphorus types can be too "chemically reactive", resulting in polishing wear. Other materials such as graphite, molybdenum disulfide, tungsten disulfide, potassium-borate and even chlorine are also used as EP agents.
There are two main types of EP additives, those that are temperature-dependent, and those that are not. The most common temperature-dependent types include boron, chlorine, phosphorus and sulfur. They are activated by reacting with the metal surface when the temperatures are elevated due to the extreme pressure. The chemical reaction between the additive and metal surface is driven by the heat produced from friction.
Rust & Corrosion Inhibitors
Rust and corrosion are caused by the attack of acidic bi-products on unprotected metal surfaces in the presence of water and oxygen. Unfortunately, these elements are present in abundance in internal combustion engines.
Rust & Corrosion Inhibitors form a protective barrier over component surfaces to seal out water and oxygen and other “oxidizers” that can cause chemical reactions to occur that lead to damage to the surface of a material.
While most rust and corrosion inhibitors work by forming a physical barrier on the component surface, some rust inhibitors function by neutralizing acids.
Bulk Oil Additives
Bulk Oil additives affect the rheological, interfacial and chemical properties of the lubricant.
Detergents
Combustion causes carbon build-up and deposit formation on the pistons, rings, valves and cylinder walls negatively affecting engine temperature and performance, oil circulation, and fuel efficiency. Some combustion by-products slip past the piston rings and into the motor oil, which can clog the engine’s oil channels.
Detergents clean engine surfaces where deposits may be detrimental. They help suspend and disperse contaminants in the oil to keep them from accumulating on component surfaces which can lead to the formation of sludge and other deposits.
Detergents also protect surfaces against carbon and varnish buildup. Additionally, they also work to neutralize acids and the corrosive effects of combustion and oxidation bi-products.
Detergents include primarily metallic salts such as calcium, magnesium and sodium.
They are most efficient at controlling high-temperature deposits.
Dispersants
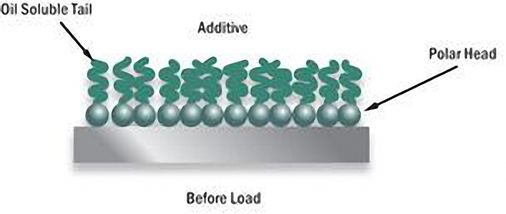
While detergents help minimize the amount of combustion by-products, dispersants hold those contaminants suspended and dispersed in the oil keeping engine surfaces free of sludge and deposits. The Larger suspended particles are removed by the oil filter.
Dispersants minimize the tendency of contaminants to agglomerate into large lumps which settle out as sludge or varnish. Although both detergents and dispersants act as cleanliness agents, they differ chemically.
Dispersants are non-metallic materials and are more effective than metallic detergents at controlling deposits under intermittent or low-temperature operations.
Additive polarity is defined as the natural directional attraction of additive molecules to other polar materials in contact with oil. In simple terms, it is anything that water dissolves or dissolves into water.
Dispersants are attracted to contaminants, such as dirt, soot, and water. They will cling to the particle and envelop it, preventing it from agglomerating and forming deposits on surfaces. They will then settle to the bottom of the oil pan or be filtered out by the oil filter, depleting your additive package.
Dispersants also fight the build-up of corrosive acids and are most efficient at controlling low-temperature deposits.
Antioxidants
Oxidation is a degradation process that occurs when atmospheric oxygen reacts with organic molecules. Like any chemical, oils can undergo chemical reactions that change the structure of molecules. For the most part, such reactions are undesirable because they lead to a change in the properties of the lubricant such that it is less effective at reducing friction and preventing corrosion.
Most hydrocarbon-based motor oils are susceptible to degradation by oxygen. This oxidation process, which is initiated by the formation of reactive free radicals and peroxides, is the major cause of oil thickening and the formation of sludge and varnish in many applications. The rate of oxidation is dependent on the quality and type of base oil as well as the additive package used. The rate of oxidation also increases with time.
Some synthetics, such as the polyalphaolefins (PAO) used in the formulation of AMSOIL's base oils, have inherently better oxidation stability than do mineral oils. This improved oxidation stability accounts for the slightly higher operating temperatures that these synthetic oils can accommodate.
Oil oxidation also produces acidic gases in the crankcase which, when combined with water, leads to corrosion and rust.
Antioxidants allow lubricants to operate at higher temperatures than would otherwise be possible without them. Excessive heat causes the oil to chemically break down which results in permanent thickening and degradation of the oil and the formation of sludge. The service life of a lubricant is greatly reduced with increases in temperature. For every 10°C increase in operating temperature, the oxidation rate of an unprotected lubricant almost doubles.
Antioxidants are additives designed to prolong the life of a lubricant by increasing the oxidative resistance of the base oil. They work to limit the impact of oxidation by reducing the tendency for oil to react with oxygen. They also retard oil oxidation reducing sludge buildup.
There are two types of antioxidants: primary antioxidants and secondary antioxidants.
Primary antioxidants are typically comprised of aromatic amines and hindered phenolics. Secondary antioxidants are typically comprised of phosphites and certain sulfur-containing compounds, such as thioethers and thioesters. Each type of antioxidant performs a different function to inhibit oxidation.
Antifoamant Additives / Foam Inhibitors
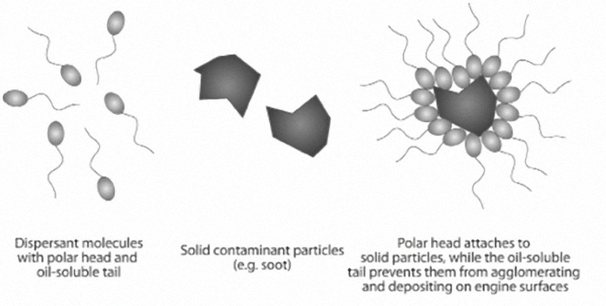
When air bubbles are whipped into motor oil by the action of the many rapidly moving parts, it results in a mass of oily froth that has very little ability to lubricate or cool the engine.
Foaming can lead to inadequate lubrication, cavitation and mechanical failure.
Antifoamant additives weaken the air bubbles, reducing their surface tension, causing them to collapse almost immediately upon forming and allowing the oil to continue protecting the engine.
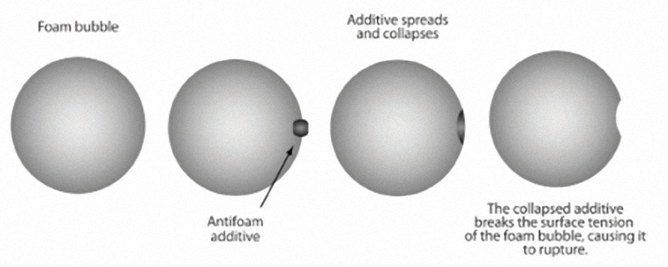
Seal Swell Agents
Motor oil must be compatible with the various seal materials used in engines. It must not cause seals to shrink, crack, degrade or dissolve.
Seal Swell agents cause seals to expand or swell slightly to ensure continued proper sealing in order to prevent leaks.
Pour Point Depressants (PPDs)
Pour Point is an indicator of the ability of oil to flow in sub-zero operating temperatures. It is the lowest temperature at which the fluid will flow. A pour point depressant lowers that temperature.
Modern refining techniques remove most of the wax (paraffin) from petroleum oil, but some wax molecules remain. These wax molecules are soluble at ambient temperatures above freezing, but crystallize and agglomerate at lower temperatures effectively increasing the oil’s viscosity, causing oil pumpability and circulation problems.
Pour Point Depressants are designed to inhibit the formation of these wax crystals, minimizing viscosity increase, and prevent them from agglomerating or fusing together at freezing temperatures.
This in turn lowers the temperature at which the oil will pour and flow. The result is easier starting, less engine wear and longer engine life.
Pour Point Depressants are found in most motor oils designed for cold weather use.
Viscosity Index Improvers (VII)
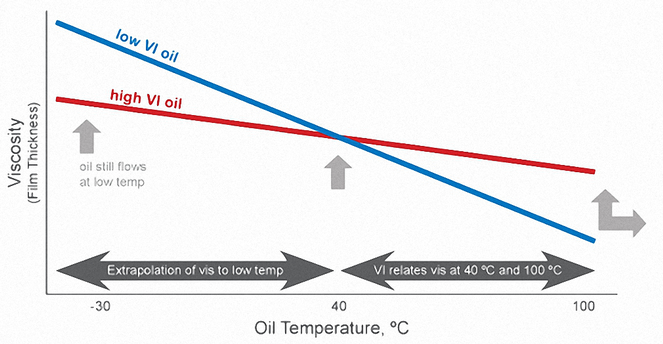
The viscosity (thickness) of a liquid is a measure of its internal resistance to flow – the thicker the oil, the higher its viscosity.
The viscosity of oil is affected by temperature changes during use. At hotter temperatures, the oil becomes thinner (viscosity decreases) and provides less engine protection. At colder temperatures, the oil thickens (viscosity increases) and becomes more difficult to pump around the engine, resulting in less protection at start-up.
The Viscosity Index (VI) is a measure of how much the oil's viscosity changes with changes in temperature. The higher the viscosity index, the less the oil’s viscosity changes with changes in temperature. In other words, oils with higher viscosity indices thin less at higher temperatures and don’t thicken as much at lower temperatures.
The Viscosity Index is simply reported as a numerical value that has no units. The measurements are taken at 40°C and 100°C.
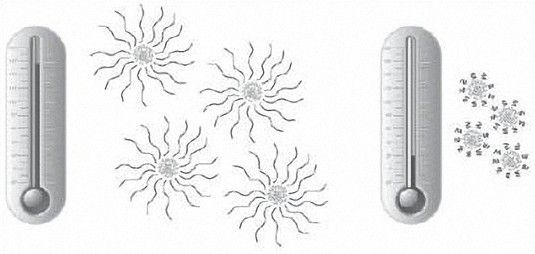
Viscosity Index Improvers (VII) are special long-chain polymers – sometimes referred to as Viscosity Modifiers (VM) – that are added to motor oil to prevent it from thinning as much as it normally would as it gets hotter. In other words, VII slow down the rate at which oil thins out as the temperature rises.
The VII polymers expand and contract as temperatures vary. High temperatures cause the molecules to expand and reduce oil thinning; low temperatures cause them to contract and have little impact on oil viscosity (see picture below).
Viscosity Index Improvers come in different shapes, sizes and quality levels (i.e. some polymer chemistries are better thickeners than others). Generally speaking, larger molecules are better thickeners than smaller ones; however, they are also more easily broken, which impacts the shear stability of the oil.
An example of a VII is an Olefin Co-polymer (OCP) which is a co-polymer of ethylene and propylene.


Home Page | Products | About AMSOIL | Oil Basics | Buy Wholesale | FAQs | Contact Us | General Site Map