Motor Oil Basics: Base Oil Groups - Select Synthetics - AMSOIL Authorized Dealer
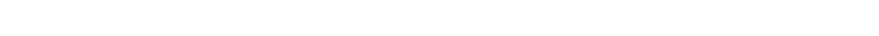


Main menu:
- Home Page
-
Products
-
AMSOIL Products
- Shop by Product
- Shop by Equipment
- Shop by Category
-
Product Lookup Guides
- Product Lookup Guides - Home
- Filter Lookup Guides
- Auto & Light Truck Lookup Guide
- Motorcycle & Dirt Bike Lookup Guide
- Harley-Davidson Products Guide
- All-Terrain Vehicle Lookup Guide
- Utility Terrain Vehicle Lookup Guide
- Snowmobile Lookup Guide
- Marine Outboard Lookup Guide
- Personal Watercraft Lookup Guide
- Small Engine Lookup Guide
- Free Product Catalog
- AMSOIL - The Blog
- AMSOIL YouTube Videos
- AMSOIL Performance Tests
- The AMSOIL Newsstand
- AMSOIL Testimonials
- AMSOIL Guarantee
- Safety Data Sheets
- Oil Analysis Services
- Shipping & Product Return
- Safe & Secure Shopping
-
AMSOIL Products
- About AMSOIL
-
Oil Basics
- What Is Motor Oil?
- What Is Oil Viscosity?
- What Does Motor Oil Do?
- Why Does Oil Need To Be Changed?
- How Often Should I Change My Oil?
- When Should I Do My 1st Oil Change?
- Can I Mix Different Oils?
- Lubrication Regimes
- Newtonian vs. Non-Newtonian
- Base Oil Groups
- Motor Oil Specifications
- Service Classifications & Grades
- Oil Evaluation Tests
- Oil Filtration
- What is Oil Analysis?
- Why Synthetics?
- Buy Wholesale
- FAQs
- Contact Us
Oil Basics


Base Oil Groups
Base Oil Classification System
The American Petroleum Institute’s (API) Base Oil Classification System classifies base oils into five major Groups based on the level of Saturates, Sulfur and Viscosity Index. Before all the additives are added to the mixture, lubricating oils begin as one or more of these five API groups.
The first three Groups are refined from petroleum crude oil. Group IV base oils are fully synthesized poly-alpha-olefin (PAO) oils. Group V is for all other base oils not included in Groups I through IV.
In general, the chemical composition and performance properties of the base oil categories improve with advancing group number. For instance, Group I base oils have a lower concentration of saturates (saturated molecules) than Group II base oils, while Group II base oils have a lower concentration of saturates than Group III base oils, and so on.
The higher the number of Saturates, the higher the molecular bond strength of the oil and therefore the better the resistance to breakdown or loss of viscosity; the lower the Sulphur content, the better the purity and thus the lower the corrosive and oxidation potential; the higher the Viscosity Index, the less the base oil’s viscosity will change with changes in temperature and therefore less Viscosity Index Improver (VII) additives will be required, the more shear stable it will be, and the longer it will last.
Today, base oils from Groups III, IV and V are considered “synthetic”. However, only base oils from Groups IV and V are truly 100% synthetic. *
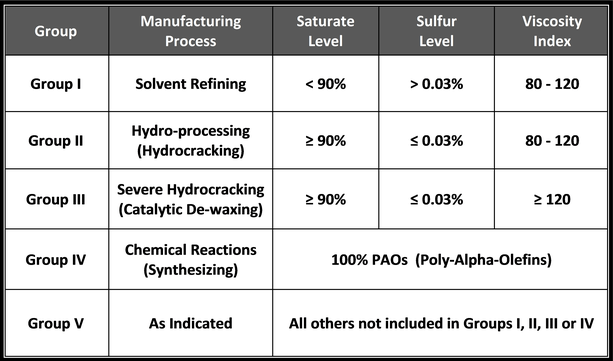
Saturated molecules contain a higher percentage of carbon-hydrogen (CH) bonds, limiting the available sites to which other, harmful molecules can attach. When other molecules, like oxygen, attach to oil molecules, they break down the molecular composition of the oil and weaken its performance.
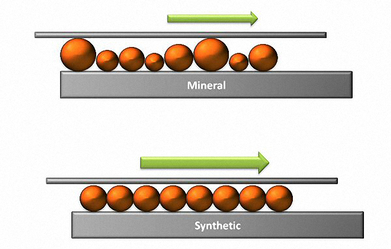
Saturated molecules are beneficial in lubricating fluids because they remain stable longer, resulting in a more durable lubricant. Unsaturated molecules have fewer single carbon-hydrogen bonds and are therefore less stable. Petroleum base oils contain far fewer saturated molecules than synthetic base oils.
Sulfur is a naturally occurring, inorganic element that readily reacts with oxygen molecules and is detrimental to oil performance. Groups III base oils contain much less sulfur than Groups I and II oils.
Viscosity Index (VI) refers to the temperature-viscosity relationship of lubricating fluids. It is an arbitrary measure of the variation in the viscosity of oil due to changes in temperature. The VI is simply reported as a numerical value that has no units. The measurements are taken at 40°C and 100°C.
Oils with a Viscosity Index less than 120 (Groups I & II) are more susceptible to viscosity variance due to temperature. Oils with a high VI are less affected by temperature changes than those with a low VI. The Viscosity Index of synthetic base oils (Groups IV & V) is much higher than that of petroleum base oils.
Group I – Solvent Refining
Group I base oils are conventional base oils manufactured by Solvent Refining, which is a much simpler refining process than Hydro-processing. Containing more than 0.03% Sulphur and less than 90% Saturates, they are less pure than Hydro-processed or synthetic base oils. The temperature range for these oils is from 0°C to 65°C and they have a Viscosity Index ranging from 80 to 120.
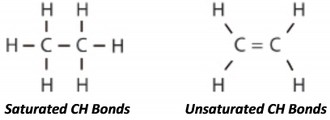
Solvent Refined base oils are usually a mix of different hydrocarbon molecules with little or no uniformity. Since the refining process cannot distinguish such molecules, the result is a lubricant with a wide assortment of molecules of different shapes and sizes, resulting in irregular lubricant surfaces at the molecular level. These irregularities generate friction within the fluid itself which increases power requirements and reduces efficiency.
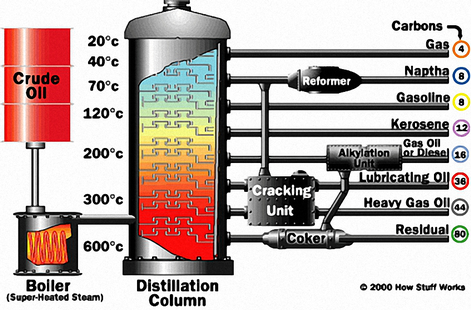
Initially, light oils such as gasoline, diesel, etc., are separated from crude petroleum by atmospheric distillation. The resulting material is charged to a vacuum distillation tower, where lubricant fractions of specific viscosity ranges are taken off. These fractions are then treated individually in a solvent extraction tower.
A solvent is mixed with them and extracts about 70-85% of the aromatic material present. Common and suitable extractants are phenol, furfural and sulfur dioxide. Furfural is used extensively as the extractant for the refining of paraffinic oils. The resulting base stocks are raffinates (referred to as neutral oils) and an extract fluid that is rich in aromatic content, which is highly sought after as process oils and fuel oils.
The solvent extracted lube fractions (raffinates) are then de-waxed by chilling to a low temperature, which removes much of the wax (paraffin). This improves the low temperature fluidity of the product. Finally, the de-waxed lube fractions are sometimes “finished” to improve their colour and stability, depending on the application requirements.
The most common method of finishing is hydro-finishing. The final quality of the base oil is determined by the severity of the application of temperatures and pressures in the hydro-finishing process. The base oils are now ready to be selectively blended with the appropriate additives.
However, even after aggressive solvent-based refining, thousands of hydrocarbon compounds - as well as organic compounds of oxygen, sulfur and nitrogen - remain. These three compounds in particular are problematic because they enable oxidation and acid development, as well as facilitate the formation of sludge, particularly in high-temperature applications.
Group I base oils are the least refined of all the groups and the cheapest base oils on the market. They make up most of the base oil produced in the world today. While some automotive oils on the market use Group I base stocks, they are generally used in less demanding applications.
Group II – Hydro-processing / Hydrocracking
With Sulphur content of less than 0.03% and Saturates content of more than 90%, Group II base oils are more pure than Group I base oils and also have a clearer color. Additionally, since Group II base oils contain a higher percentage of saturates than Group I oils, they have better antioxidation properties.
However, like Group I base oils, they also have a low viscosity index (ranging from 80 to 120).
Group II base oils are manufactured by what the API calls Hydro-processing or Hydrocracking, which is a much more complex process than the Solvent Refining process used for Group I base oils.
Cracking, as the name suggests, is a process in which large hydrocarbon molecules are broken down into smaller and more useful ones.
There are significant differences in performance between Hydrocracked and Solvent Refined base oils. The main reason for the difference lies in the virtual elimination of aromatic molecules (typically less than 0.1%) in the Hydrocracked base oils. In comparison, the aromatic content of Group I Solvent Refined base oils is typically between 10-30%.
There are two stages in the Hydrocracking process. The first one removes unwanted polar compounds and converts the aromatic components to saturated hydrocarbons. With the Hydrocracking process, the elimination of aromatics and polar compounds is achieved by reacting the feedstock with hydrogen in the presence of a catalyst at high temperatures and pressures.
Catalysts are substances that speed up reactions by providing an alternative pathway for the breaking and making of bonds. Key to this alternative pathway is a lower activation energy than that required for the uncatalysed reaction.
Several different reactions occur in this process, the principal ones being:
- Removal of polar compounds containing sulphur, nitrogen and oxygen
- Conversion of aromatic hydrocarbons to saturated cyclic hydrocarbons
- Breaking up of heavy polycyclo-paraffins to lighter, saturated hydrocarbons
These reactions take place at temperatures as high as 400°C/752°F, pressures around 3000 psi and in the presence of a catalyst. The hydrocarbon molecules that are formed are very stable and this makes them ideal for use as lubricant base oils classified by the API as Group II base oils.
After separation into desired viscosity grades by vacuum distillation, batches of waxy lube base oil are chilled and de-waxed. These are then passed through a second high pressure hydro-treater for additional saturation. This final step maximizes base oil stability, by removing the last traces of aromatic and polar molecules.
There is no question about the higher purity of hydro-treated base oils, but they do have some disadvantages. Some additive types cannot be effectively blended with these base oils because they drop out of solution. That is, hydro-treated base oils cannot retain their solubility for some chemicals, and thus additive retention may be seriously affected.
In addition, because severely hydro-treated base oil contains almost no aromatics, these oils must be fortified with seal swell agents in the additive package. Solvent-refined base oils, on the other hand, retain some aromatics, which are “natural” seal swell agents.
Group II base oils are very common on the market today and are priced very close to Group I base oils.
Group III – Severe Hydrocracking (and Hydro-isomerisation)
Group III base oils contain greater than 90% Saturates, less than 0.03% Sulfur and have a Viscosity Index (VI) of 120 and above. The main difference between Group II and III base oils is that the Group III base oils have a higher VI.
Group III base oils are refined even more than Group II base oils and are severely hydrocracked (higher pressures and heat). This longer more involved process is designed to achieve a higher quality and more pure base oil.
Although still derived from petroleum/crude mineral oil, Group III base oils are sometimes described as synthesized hydrocarbons (“synthetics”).*
Severe Hydrocracked base oils have the following attractive features:
- High Viscosity Index (up to 130) (they ‘thin-out’ less at high temps and ‘thicken’ less at low temps)
- Excellent Oxidation Resistance (they also respond very well to Anti-Oxidants)
- High Thermal Stability (they have very good Heat Resistance)
- Low Toxicity (due to a virtual absence of impurities)
- Excellent Low Temperature Fluidity
- Produce Low Carbon Residue (they keep engines cleaner)
- Separate Readily from Water
- Low Volatility (less evaporation/lower oil consumption)
- Good Biodegradability Characteristics (reduced Environmental Impact)
These features give performance characteristics similar to, but not quite as good as, lubricants based on Group IV Polyalphaolefins or Group V base oils.
Like Group II base oils, these oils are also becoming more prevalent today.
The Hydro-isomerisation process
The Hydro-isomerisation process employs a special catalyst to selectively isomerise wax (paraffin mixture) to high viscosity index, low pour point, isoparaffinic lube oil.
Isomerisation is the process in which hydrocarbon molecules are rearranged into a more useful isomer.
An Isomer is any of two or more compounds with the same molecular formula or weight but with different chemical structures (having different arrangements of atoms).
This process yields base oils with higher VIs (up to 130 in a single pass) and improved yields, compared to previous conventional de-waxing techniques. A further process feature is the flexibility it offers to produce base oils with ultra low pour points (lower than -25°C).
Group IV - Chemical Reactions (Synthesizing)
Group IV Polyalphaolefins (PAOs), which are made from very small uniform molecules, are chemically engineered through a process called synthesizing and therefore are considered true 100% synthetic base oils.
Polyalphaolefins provide excellent performance characteristics and have few negative attributes. The first commercial process for making PAOs was pioneered by Gulf Oil in 1951.
PAO base oils are actually similar to mineral oils. The advantage comes from the fact that they are built, rather than extracted and modified, making them more pure. Since they are “man-made”, they are tailored to have a controlled molecular structure with predictable properties.
Poly-alpha-olefins are usually highly purified ethylene derivatives and have very stable and pure chemical compositions and highly uniform molecular chains. Practically all of the oil molecules are the same shape and size. They also contain no sulfur, phosphorus, or wax.
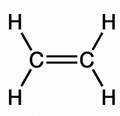
Olefins are also known as alkenes. An alkene is an unsaturated hydrocarbon containing at least one carbon-carbon double bond.
Olefins/Alkenes can participate in a wide variety of reactions. The majority of the reactions of alkenes involve the rupture of the double bond, forming new single bonds. Ethylene (C2H4), also known by the name ‘ethene’, is the simplest alkene/olefin (see illustration below).
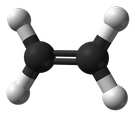
An alpha-olefin (or α-olefin) is an alkene where the carbon-carbon double bond starts at the α-carbon atom. This location of a double bond enhances the reactivity of the compound and makes it useful for a number of applications.
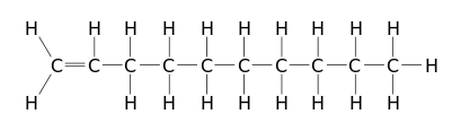
There are two types of alpha-olefins, branched and linear (or normal). The alpha-olefin most commonly used for lubricant base stocks is the 1-Decene (or α-Decene) which contains a chain of ten carbon atoms with one double bond (see illustration below).
A poly-alpha-olefin (or poly-α-olefin, abbreviated as PAO), is a polymer made by polymerizing an alpha-olefin.
Polymerization is a chemical process that combines several monomers to form a polymer by converting the double bond to a single bond and forming other single bonds to join the other monomers. Polymers from alkene/olefin monomers are referred to in a general way as poly-olefins.
PAOs have a much broader temperature range than Group I, II or III base oils and are therefore great for use in extreme cold conditions and high heat applications. They offer better protection at high temps (a thicker hydrodynamic film) and reduced energy losses and easier starting at low temps.
PAOs flow easily at low temperatures and withstand high temperatures with minimum decomposition. When combined with Group V Esters, and an additive package, they offer excellent performance over a wide range of lubricating properties.
AMSOIL’s Premium Lubricants are made up primarily of Group IV PAO base oils (with some Group V Esters).
Finished lubricants based on Group IV base stocks have:
- Extremely high native viscosity index (VI)
- Excellent low temperature fluidity
- Much wider viscosity range
- Very low pour points
- Higher flash points and fire points
- Superior volatility resistance
- Greater oxidative stability
- Excellent thermal stability
- Greater shear stability
- Better natural detergency
- Very low toxicity
- Lower traction force / internal friction
- Reduced energy consumption
Group V - As Indicated
Group V base oils include all other oils not included in Groups I, II, III or IV, including silicone, phosphate ester, diester, polyolester (POE), polyalkylene glycol (PAG), alkylated benzene, biolubes, etc. Basically, if it is a synthetic base oil and it is not a PAO, it is a Group V base oil.
Group V synthetic base oils are used primarily in the creation of motor oil additives. They are generally not used as base oils themselves, but add beneficial properties to other base oils.
Esters are common Group V base oils used in different lubricant formulations (usually as additives) to improve and enhance the properties of the existing base oil. They can take more abuse at higher temperatures than other base oils and are great for high-temperature hydrodynamic applications because they can survive in extreme environments where no other lubricant can.
Some say esters compete so vigorously for the metal surfaces that they crowd out necessary additives. However, many additives are active enough to displace an ester from a surface. Expertise and experience are important here, as some additives do not work well with synthetic esters.
Esters have outstanding oxidative stability and do not form many radical decomposition products; they tend to evaporate cleanly before undergoing oxidative polymerization so they will not form deposits and varnish. They provide superior detergency compared to PAO synthetic base oil, which in turn increases the hours of use.
Esters are generally considered good boundary lubricants because they associate with metal surfaces reducing the amount of metal-to-metal contact during sliding motion. However under severe conditions, anti-wear and extreme-pressure additives are used to carry the bulk of the load.
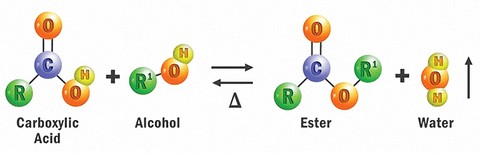
Esters are 100% synthetic chemical compounds derived by reacting an oxoacid (carboxylic acid) with an alcohol. That is, they are formed by condensing an acid with an alcohol (with the elimination of water, which is a by-product of the esterification reaction).
Organic chemists call this a reversible reaction because water can react with ester groups and break-up the ester into its individual components (acid and alcohol). This is known as hydrolysis.
There are endless varieties of esters that can be built from commonly available acids and alcohols, so almost anything is possible. Modern synthetic esters can be “tuned” to perform in nearly any environment and application. They provide almost unlimited structural and performance possibilities.
One common Ester often used in the formulation of synthetic motor oil is Diester. A Diester is an organic compound containing two ester functional groups. It has excellent oxidative and thermal stability, very high VI, and excellent solubility. It is also compatible with most lubricants in the market.
Diester is often used as an additive with Group IV PAO basestocks to provide the necessary solvency for the oil’s large additive package. As a side effect, the finished oil will have excellent detergency.
Another commonly used Ester is Polyol Ester (POE) (see illustration below). Polyolesters have several excellent performance properties, including thermal stability, super-high VI and fire resistance. Of all the base oils mentioned, they are probably the best choice for very high-temperature applications.
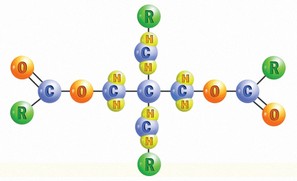
The two most common applications for Polyolesters are fire-resistant hydraulic fluids and jet engine oils, but they can also be used in the formulation of motor oils. POEs are compatible with most lubricants.
___________________________________________________
Now that said, what matters in the end, is the quality and performance of the finished blended product. The point is, an oil can’t be judged solely by its base oils – you need to take the entire formulation into account (base stock plus additives).* In 1999 the National Advertising Division (NAD) of the Better Business Bureau (BBB), in response to a complaint from Mobil, ruled that Castrol Syntec, which was based on a Group III+ base oil, could be considered "synthetic" because modern oils made using Severe Hydrocracking and Hydro-isomerization have most of the same performance features of a “true” Synthetic.In light of this “non-binding” ruling, the rest of the major oil manufacturers and blenders decided it was safe to expand that interpretation to cover all Group III base oils and proceeded to do so. It was a low risk marketing decision betting that no one would challenge the move after the NAD ruling, and they were right, no one did.As a result, the synthetic line was drawn by the oil marketers between Group II+ and Group III base oils. This took pretty much all of the meaning out of the term “synthetic”.However, only Groups IV & V base oils are truly 100% Synthetic.Note: In some European countries (e.g. Germany), unlike in North America, only Groups IV and V based motor oils can actually be labelled “Synthetic”.


Home Page | Products | About AMSOIL | Oil Basics | Buy Wholesale | FAQs | Contact Us | General Site Map